Who am I?
My name is Lorenzo Rovigatti and my email is lorenzo.rovigatti@uniroma1.it
How did I get here?
- 2009 - Laurea in Fisica & PhD in Materials Science here (with FS)
- 2013-2014 - Post-doc here (with FS)
- 2014-2016 - Post-doc at the University of Vienna
- 2016-2017 - Post-doc at the University of Oxford
- 2017-2018 - Researcher (RTDA) at CNR
- 2018-2021 - Tenure-track researcher (RTDB)
- 2021 - Associate Professor here
What are my research interests?
- Soft matter (including some biophysics)
- Self-assembly (colloids, proteins, nucleic acids)
- DNA nanotechnology
- Polymeric assemblies (star polymers, microgels)
Some pictures
Polymer networks
Polymeric objects (microgels)
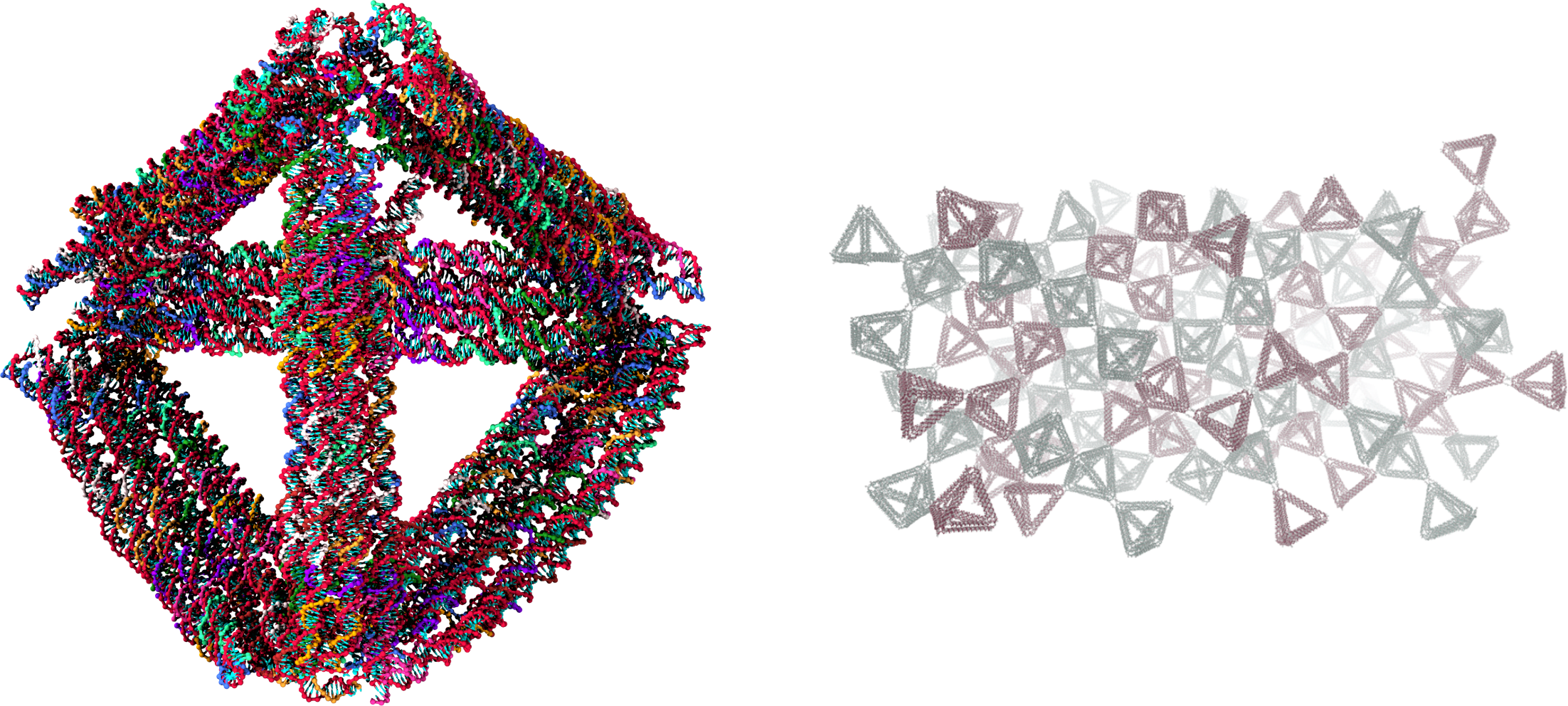
All-DNA materials
DNA nanotechnology
This is not a scientific talk at a conference, it is a lesson
Please do interrupt me to ask questions
Harnessing entropy to drive phase separation
Lorenzo Rovigatti
Physics Department, Sapienza University of Rome
Biological and Soft Matter, December 14th, 2023
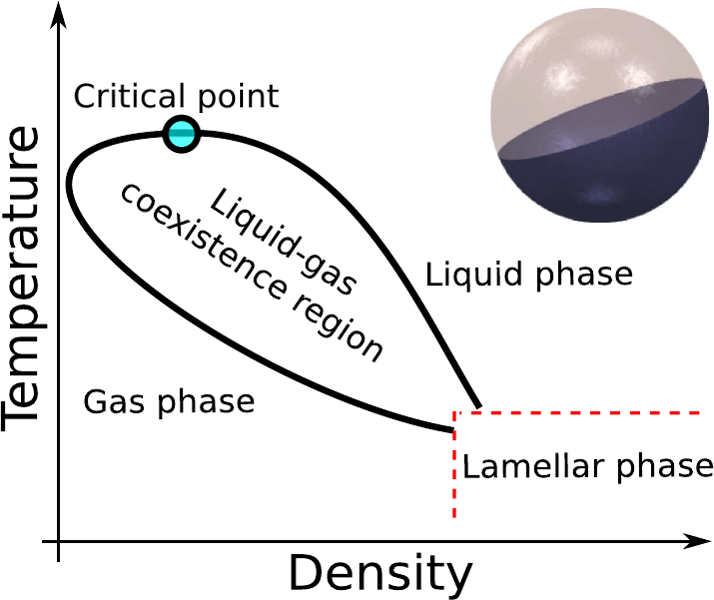
The classic, molecular "gas-liquid" picture
The Van der Waals recipe for the gas-liquid phase separation:
- A "long-range" attraction
- A "short-range" repulsion
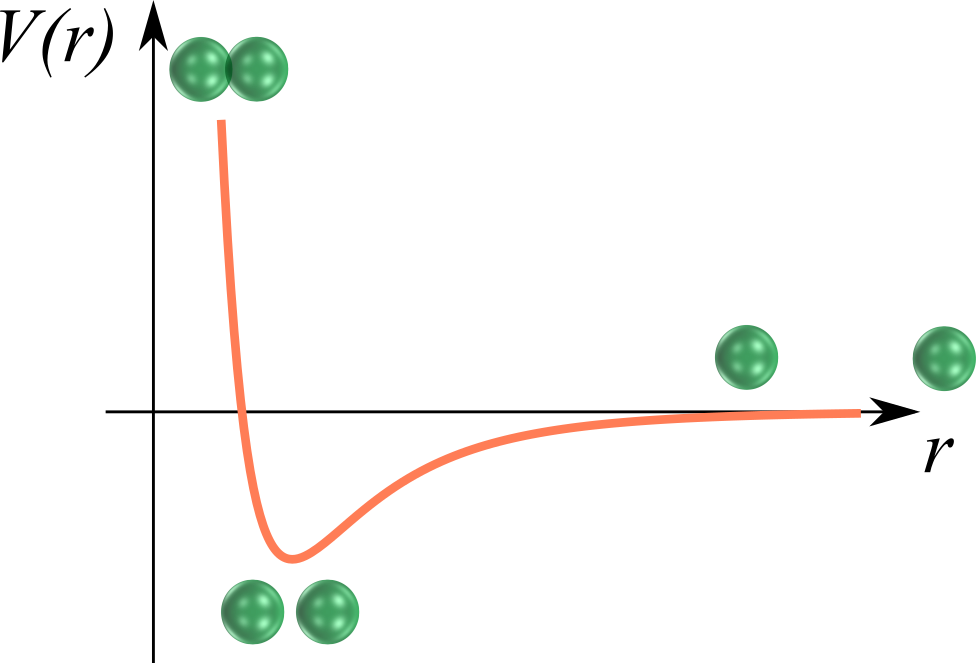
The transition takes place between two phases
- A low-density phase with high entropy $S_g$ and high energy $U_g$ (the gas)
- A high-density phase with $S_l < S_g$ and $U_l < U_g$ (the liquid)
Tuning the phase diagram
Example: the effect of the range of a spherical attraction
$\approx$ particle size

Fraction of particle size

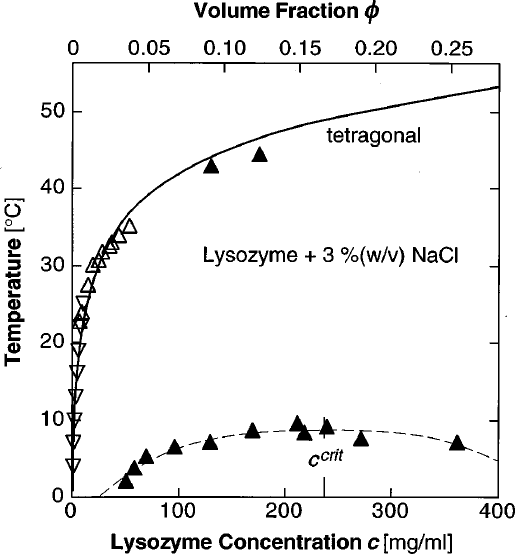
The soft-matter picture
- Particle-particle interactions can be tuned to a large extent
- The phase behaviour can (at least in principle) finely tuned
- A classic example: the depletion interaction
- Mixture of small (depletants) and big (colloids) particles
- There is only excluded volume: energy is 0 or $\infty$ $\to$ only entropy counts
- Two colloids get close $\to$ depletants have more space $\to$ higher entropy
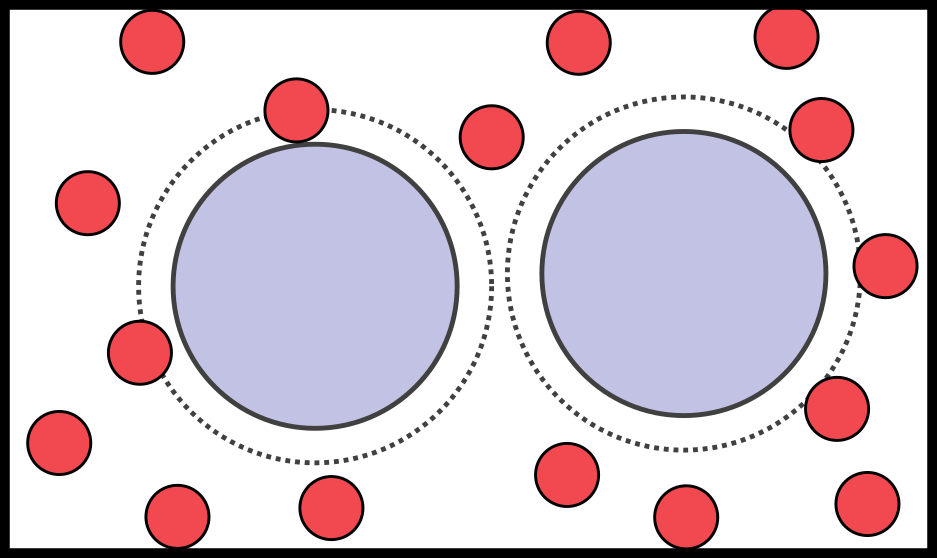
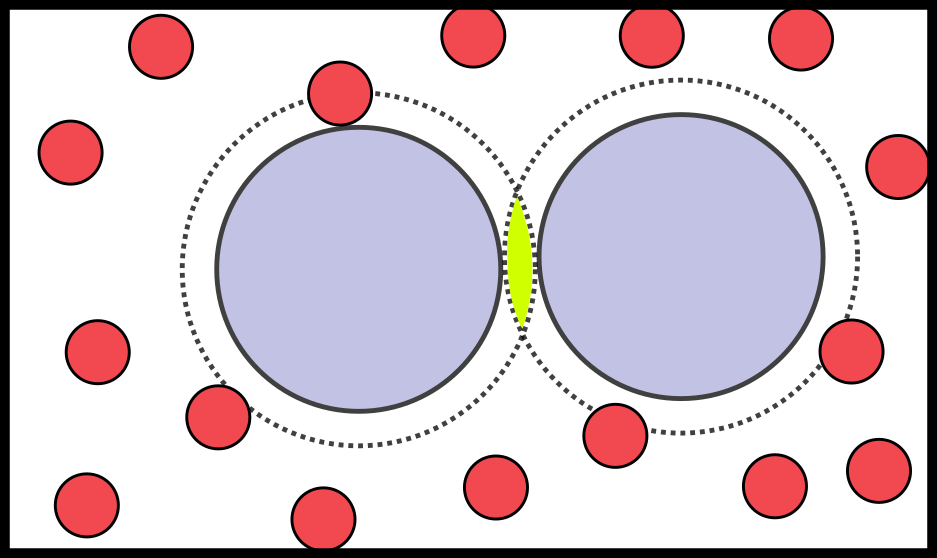
Colloids feel an effective attraction due to entropy alone
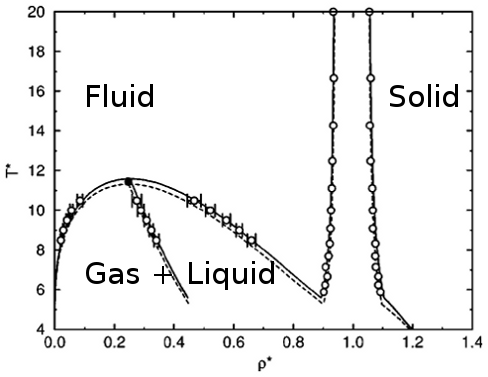
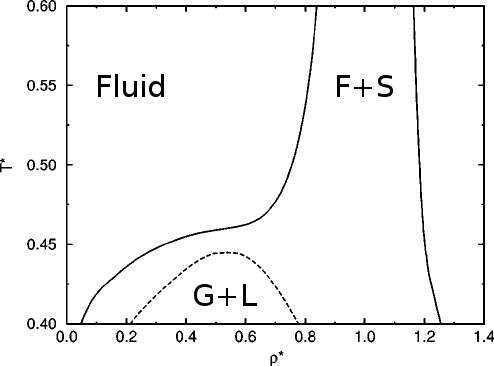
Different mechanisms, similar phase diagrams
- The "molecular" phase diagram[1]
- A depletion phase diagram[2]

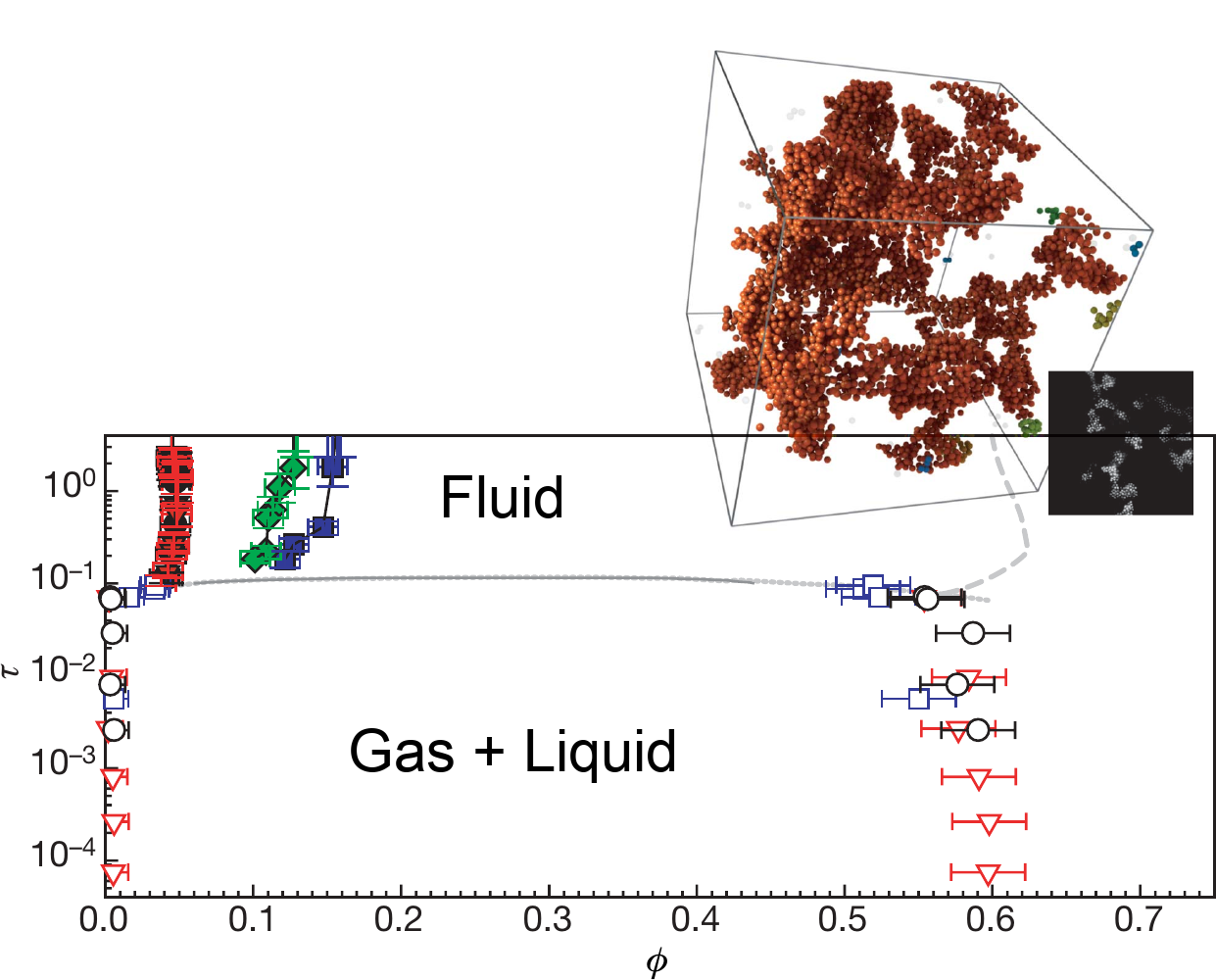
Complex behaviour can be obtained with more complicated building blocks
[1] Muschol and Rosenberger, J. Chem. Phys. (1997), [2] P. J. Lu et al., Nature (2008)
Tuning the phase behaviour of soft-matter systems
Self-assembly vs. phase separation
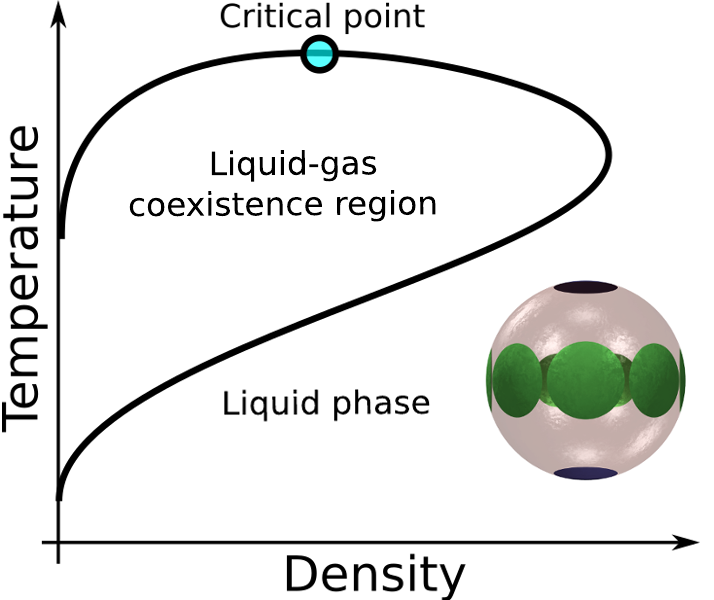
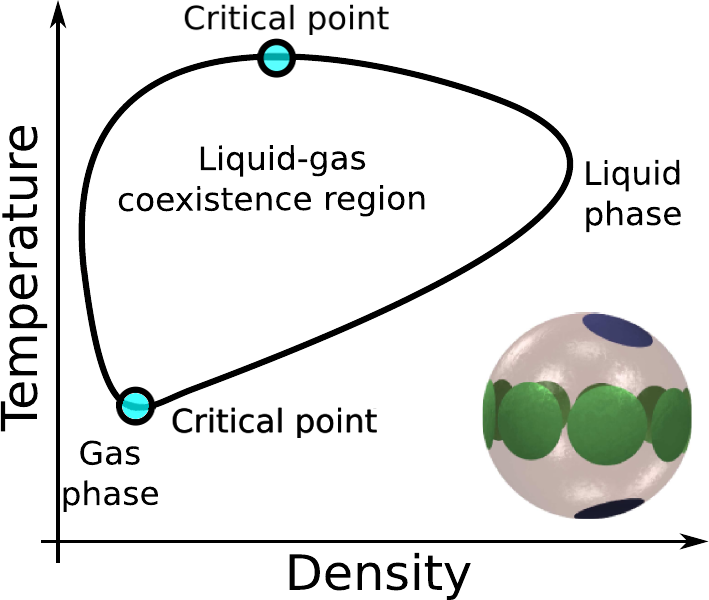
- At low temperature, Janus particles form stable micelles that stabilise the gas[1]
- Chain formation in network fluids stabilises the liquid[2]
- The two mechanisms together can produce a second critical point[3]
Increasing complexity: flexibility & deformability
Many soft-matter building blocks are polymer-based $\to$ internal degrees of freedom- Polymers (chains, rings, star polymers, etc.)
- Micron-sized polymer networks (microgels)
- DNA-based particles (DNA nanostars, DNA origami, etc.)

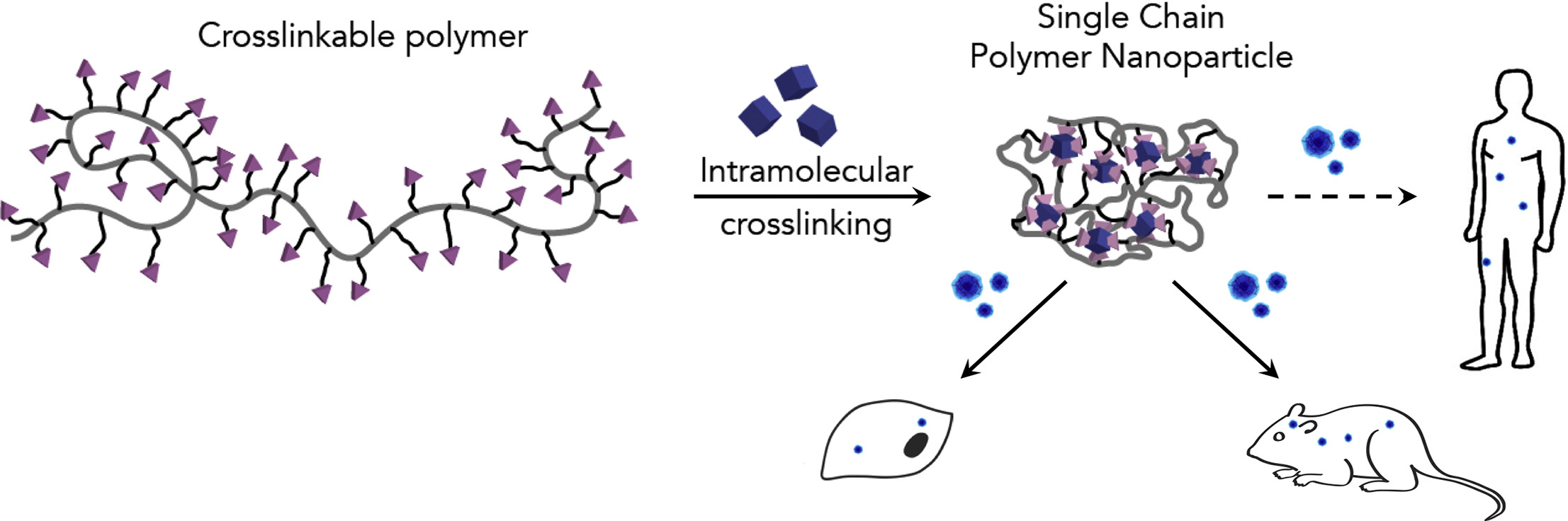
Associative polymers
- Polymers decorated with attractive monomers
- Diluted $\to$ single-chain nanoparticles (SCNPs)[1]
- Concentrated $\to$ transient polymer networks (useful as e.g. model biosystems[2])
- Strong but exchangeable bonds $\to$ covalent adaptable networks (e.g. vitrimers[3])
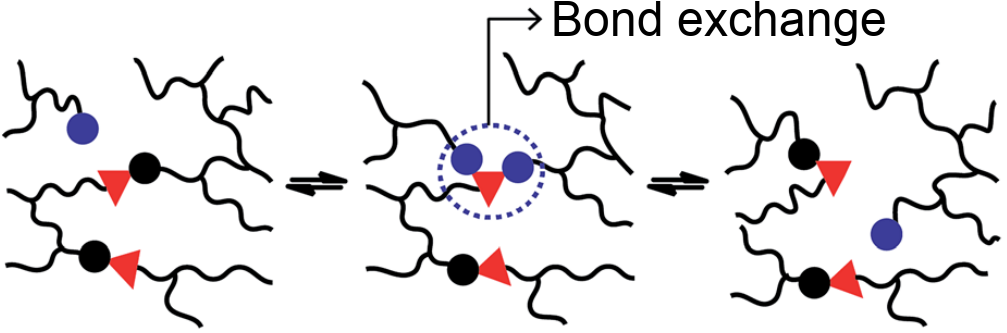
[2] J.-M. Choi, A. S. Hoblehouse, and R. V. Pappu Annu. Rev. Biophys. (2020)
[3] W. Denissen, J. M. Winne, and Filip E. Du Prez, Chem. Sci. (2016)
What contributes to the thermodynamics of associative polymers?
- I will focus on models that can form both intra- and inter-molecular bonds
- At low concentrations polymers don't meet: intra-molecular bonds are more likely
- Forming an intra-molecular bond costs entropy
- All things being equal, inter-molecular bonds are combinatorially favoured
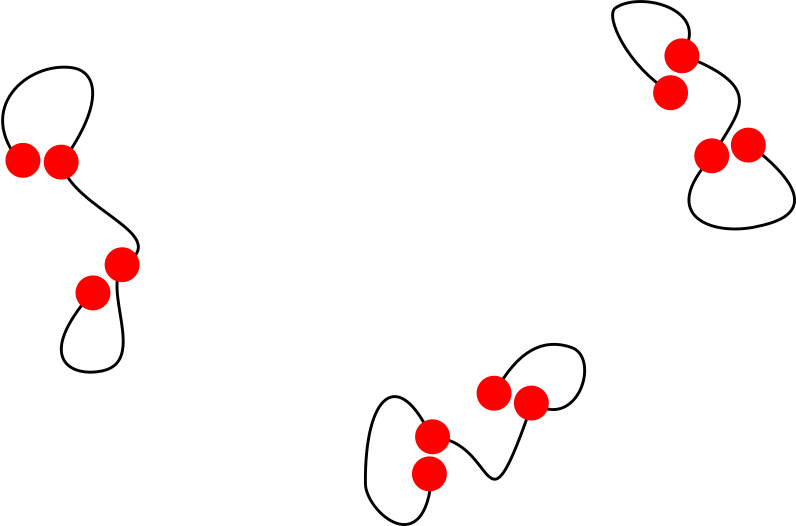
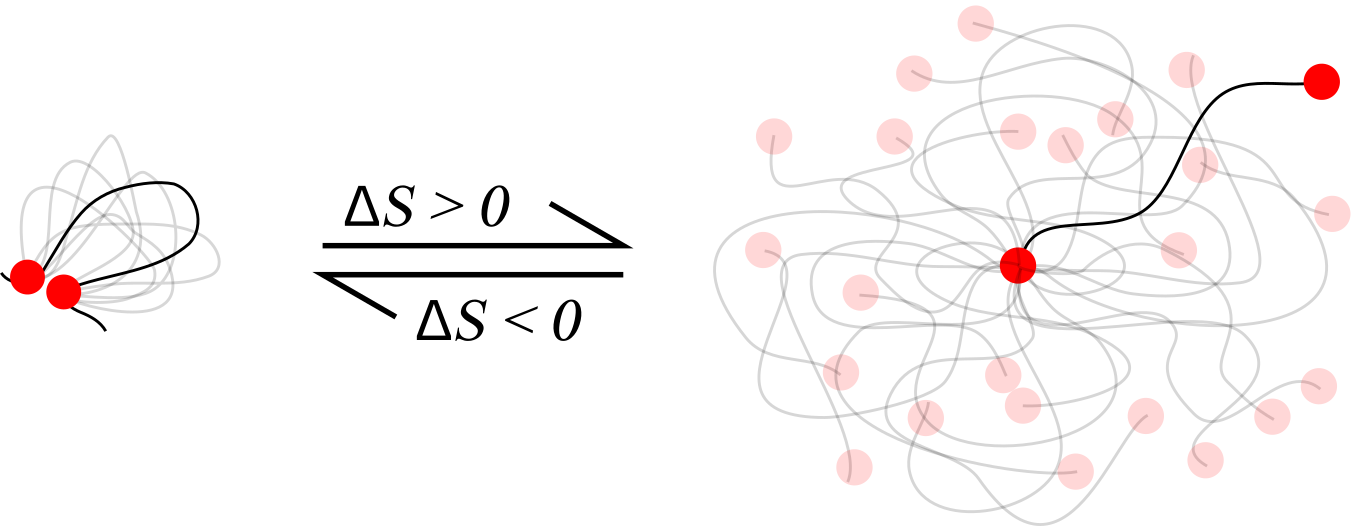
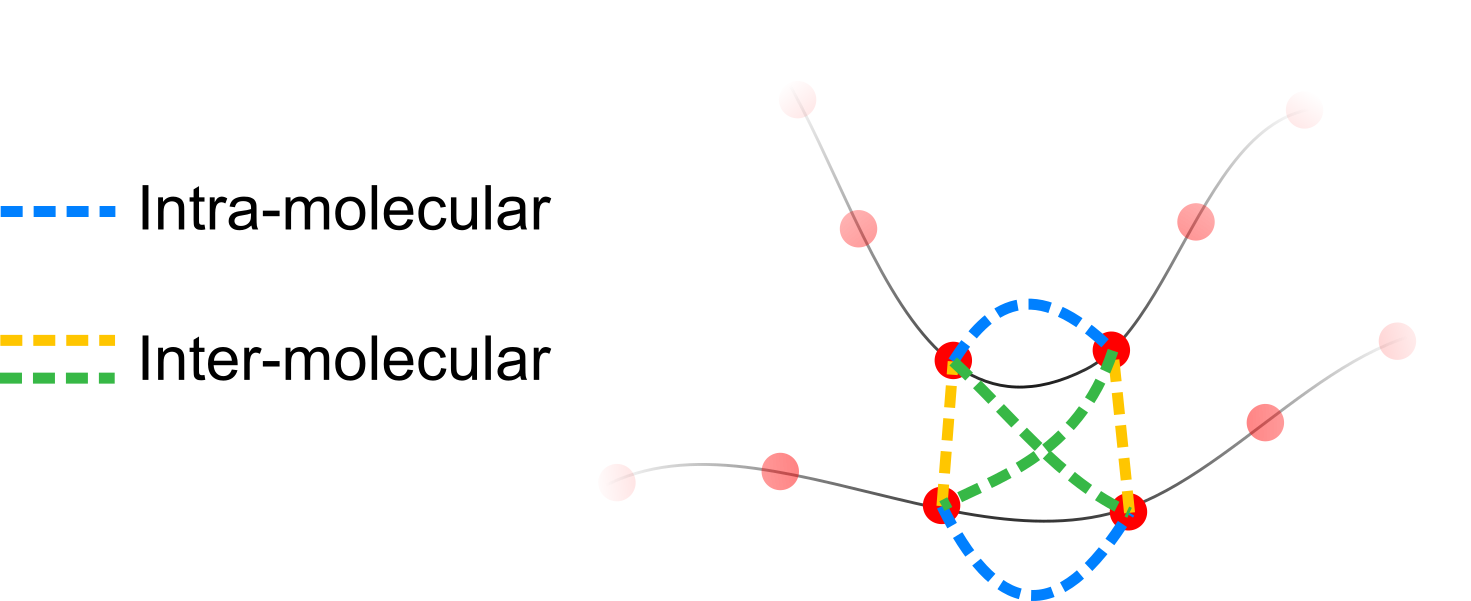
Overall, the number of inter-molecular bonds increases with density
The thermodynamics of associative polymers - results from literature
- According to theory, "simple" associative polymers do not phase separate[1]
- The conversion of intra- to inter-molecular bonds is continuous
- SCNPs start to bind to each other and form transient polymer networks
- Experiments[2] and simulations[3] confirm this picture
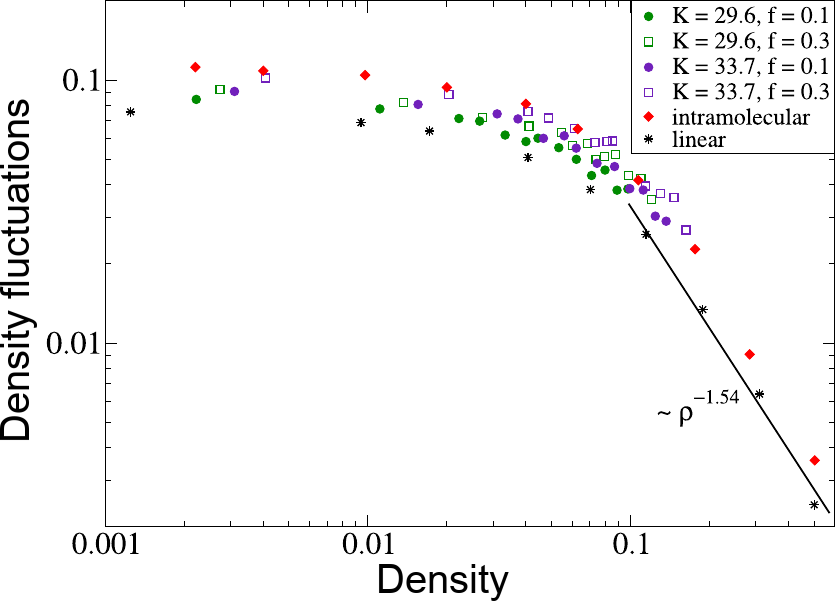
[1] A. N. Semenov and . Rubinstein, Macromolecules (1998)
[2] D. E. Whitaker, C. S. Mahon, and D. A. Fulton Angew. Chem. (2013)
[3] M. Formanek et al., Macromolecules (2021)
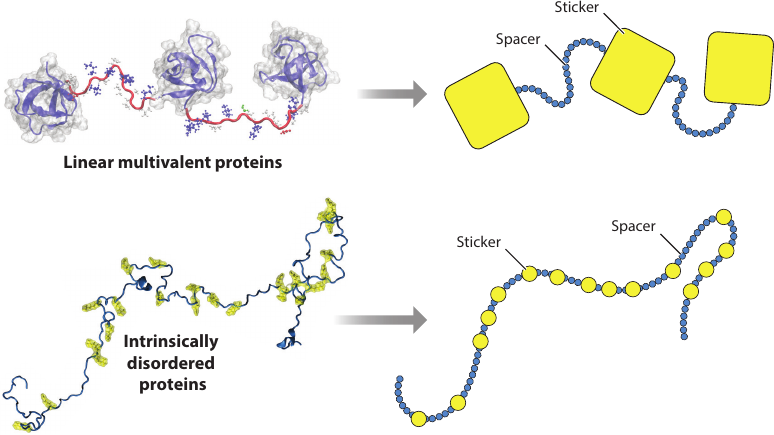
Associative polymer models
The goal: control the thermodynamics by manipulating the sequence
- Equispaced reactive monomers along the chain
- Three models: one, two or three types of (alternating) reactive monomers
- Only like-type reactive monomers can bind to each other
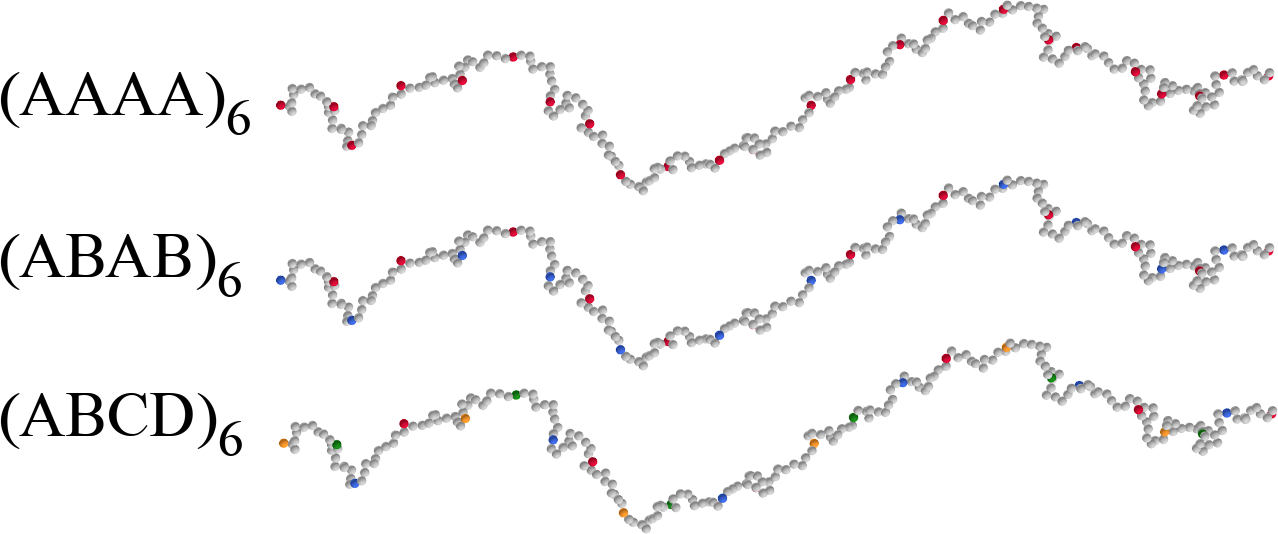
The low-density fully-bonded limit
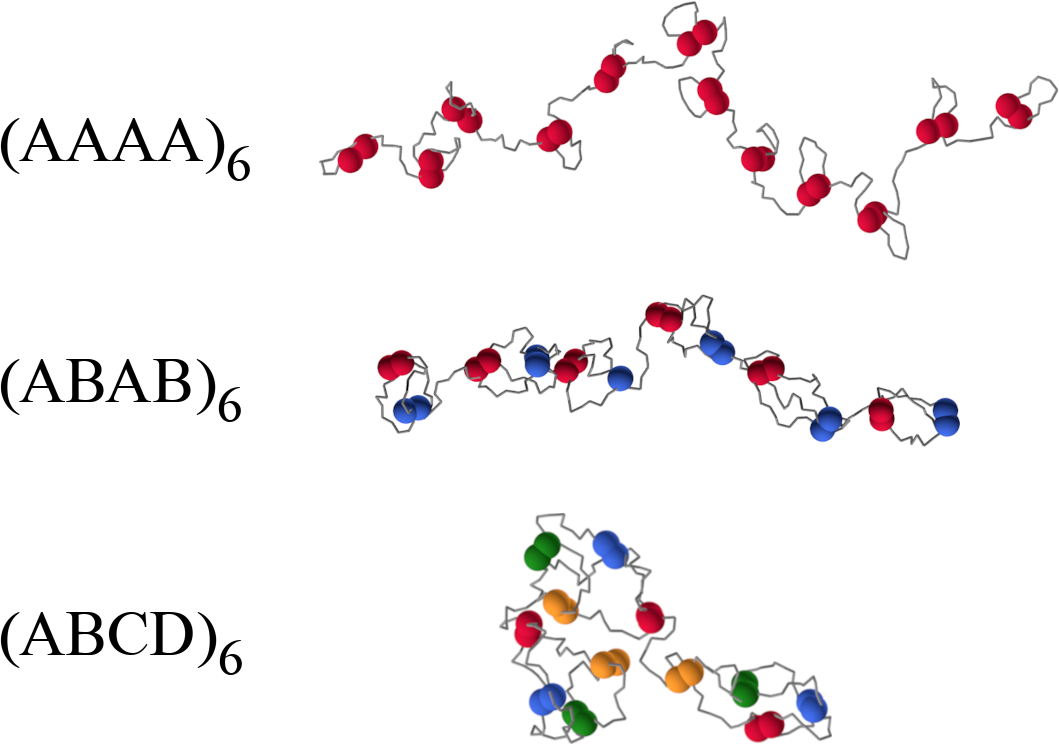
- In all three cases the energy is the same
- To satisfy all bonds, polymers have to "fold"
- Closing a loop costs entropy: the longer the loop, the higher the cost
- The largest the distance between "compatible" monomers, the longer the loop
- The cost per reactive site is $\approx -4\, k_B$ for $(AAAA)_6$ and $\approx -5\, k_B$ for $(ABCD)_6$
Effective potentials from two-chain simulations
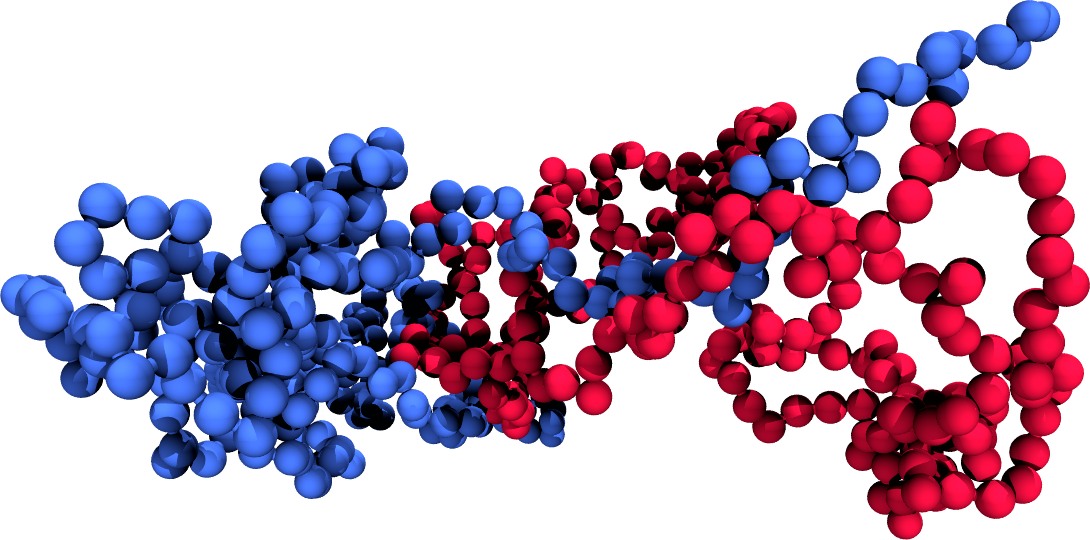
- Add a contribution depending on the chain-chain distance $R$ to $H$, $H_{\rm bias}(R)$
- Run simulations to obtain biased averages, $\langle \cdot \rangle_{\rm bias}$
- Any observable that depends on $R$ can be obtained from biased simulations as $$ \langle O(R) \rangle_{\rm unbias} = \langle O(R) \rangle_{\rm bias} e^{\beta H_{bias}(R)} $$
- If necessary, run simulations with different biases and "glue" the results together, recalling that $U_{\rm eff}(R) = -k_B T \log(g(r)_{\rm unbias})$
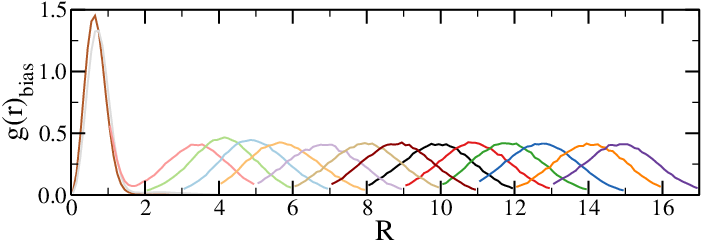
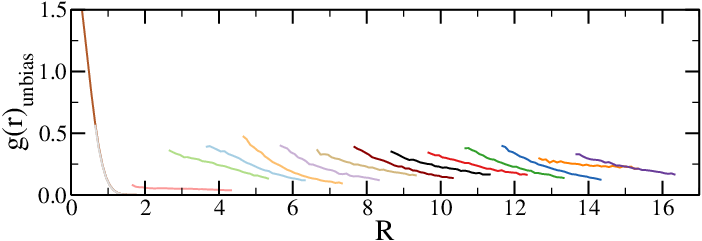
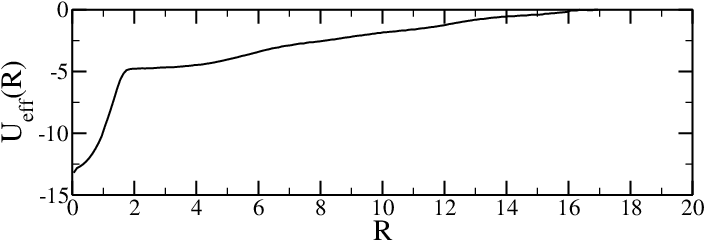
Effective potentials from two-chain simulations

- All bonds are formed $\to$ the only contribution is entropic
- Only intra-molecular bonds $\to$ strong repulsion
- Intra- and inter-molecular bonds $\to$ from weakly repulsive to strongly attractive
- In the $(AAAA)_6$ system, $\beta V \approx 0$ $\to$ attraction $\approx$ repulsion
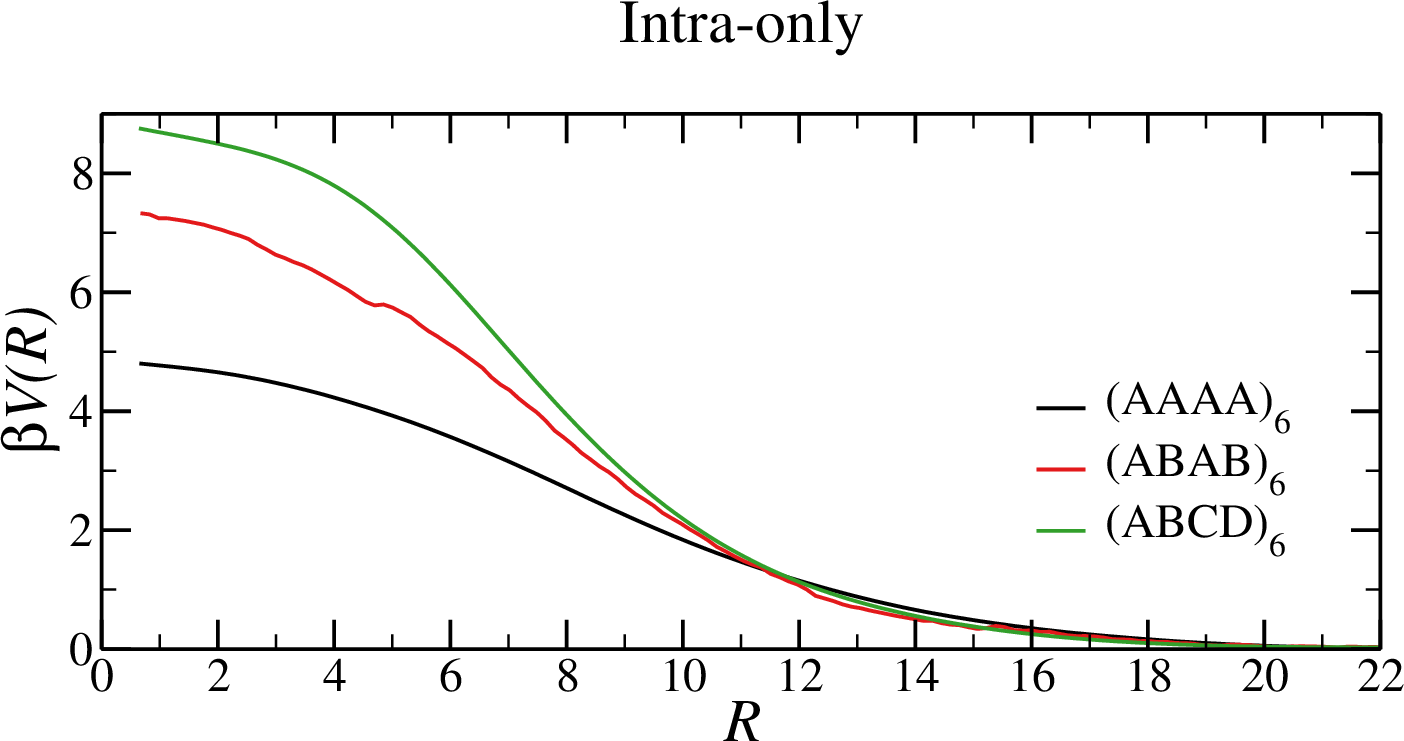
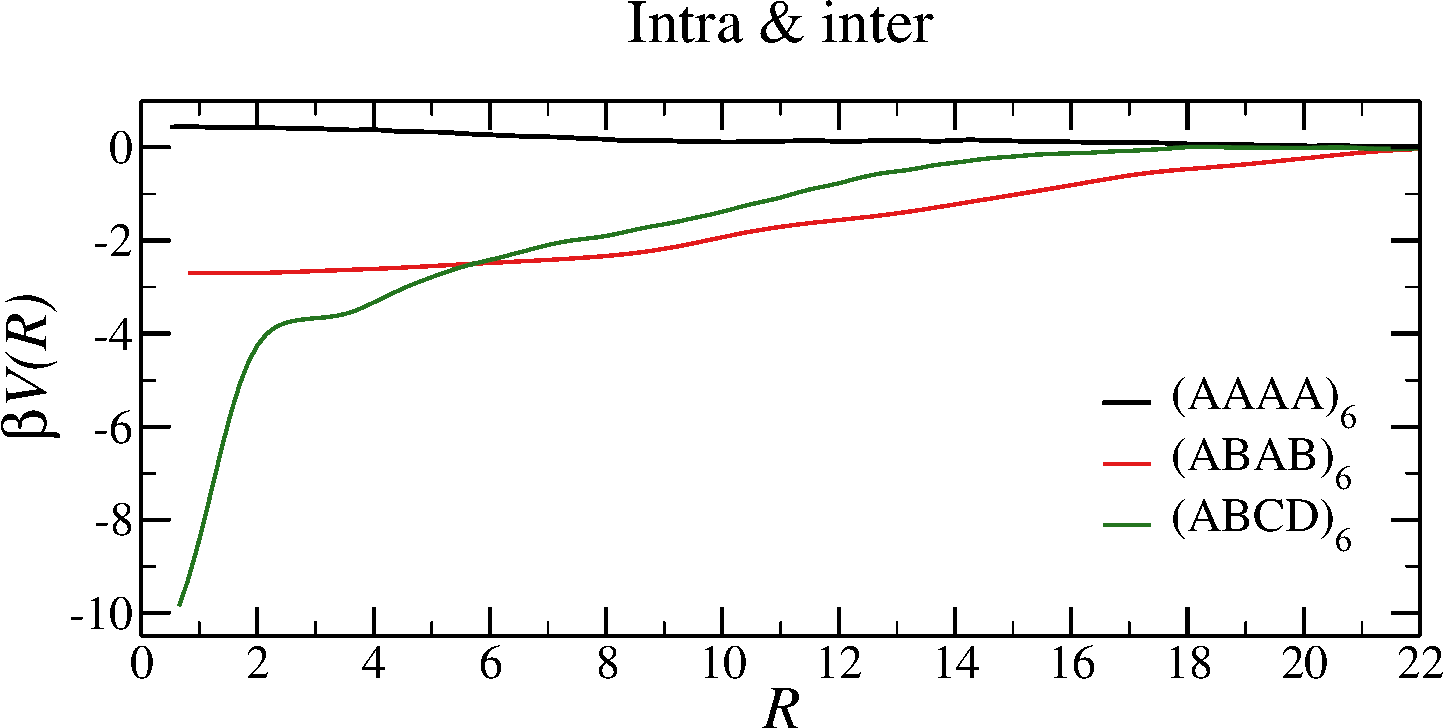
Equations of state in the fully-bonded limit
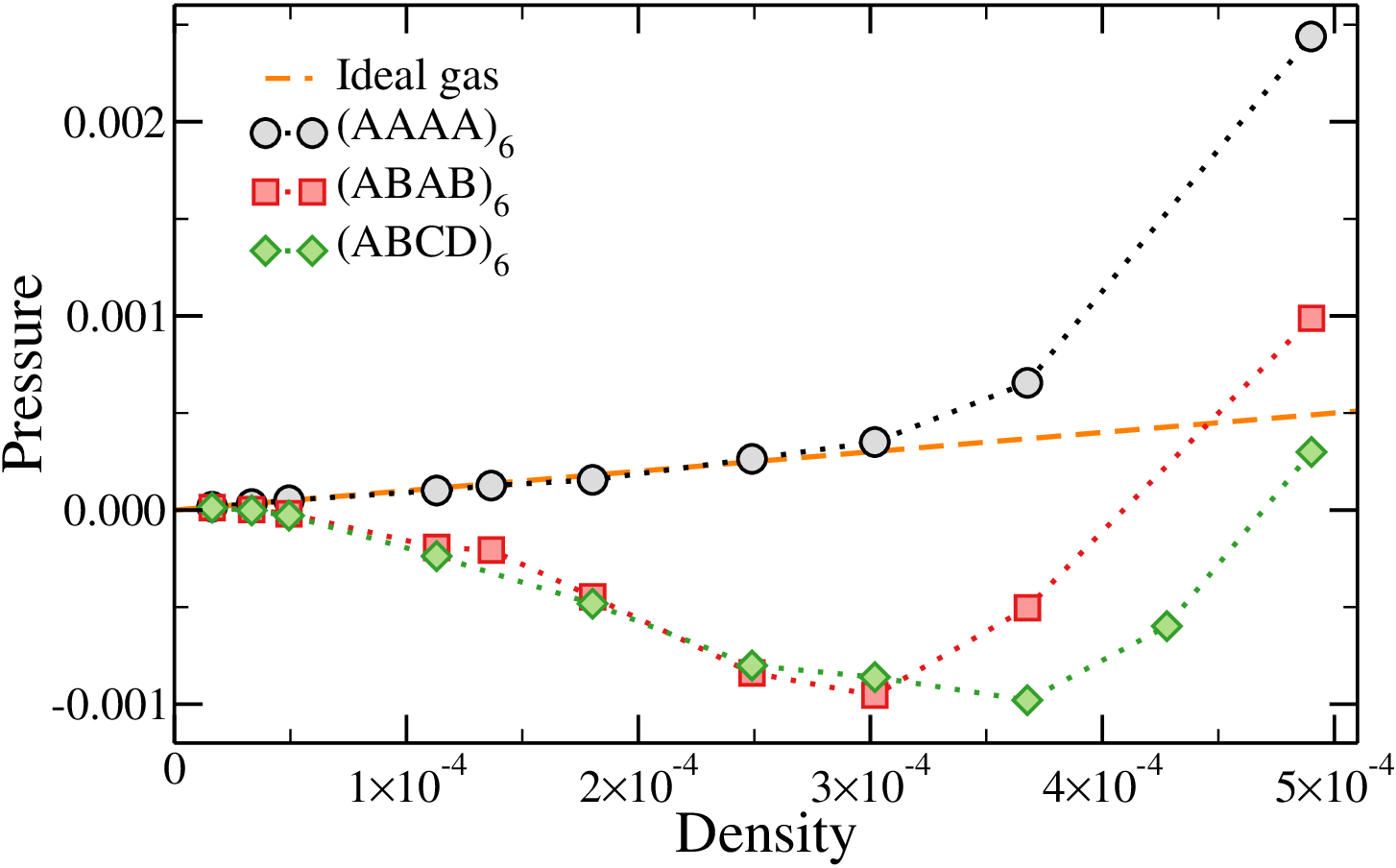
- The $(AAAA)_6$ behaves like an ideal gas in a large density range
- Both "designed" systems display a non-monotonic equation of state
Signature of phase separation?
Signature of phase separation!
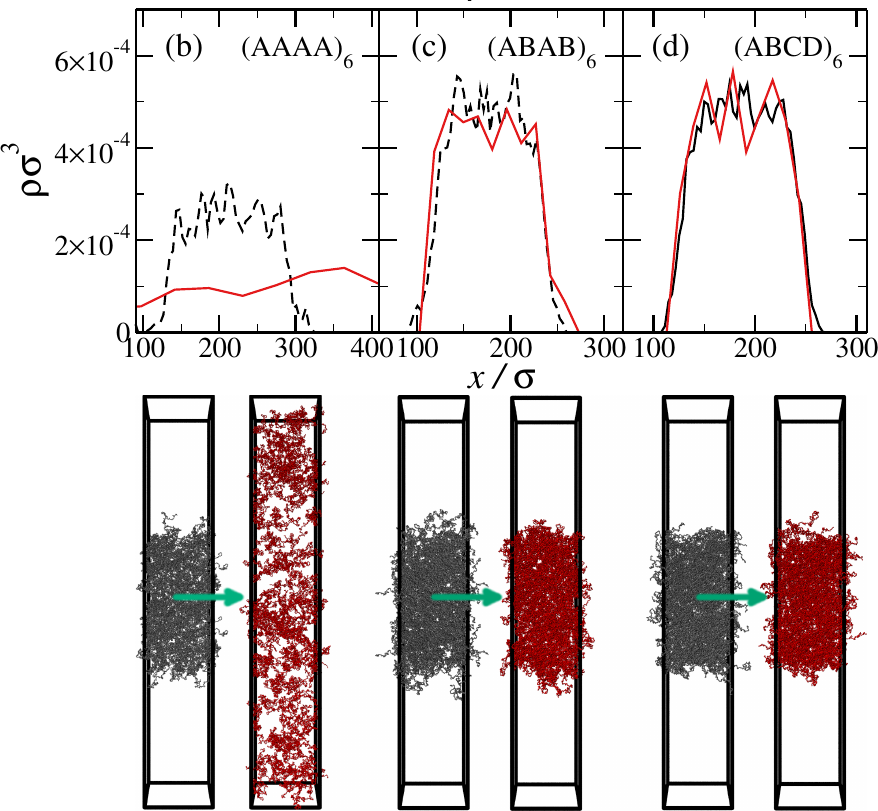
- In the $(AAAA)_6$ system, the interfaces melt
- In the designed systems, the density remains heterogeneous
Phase diagram of the (ABAB)$_6$ model
Direct-coexistence simulations
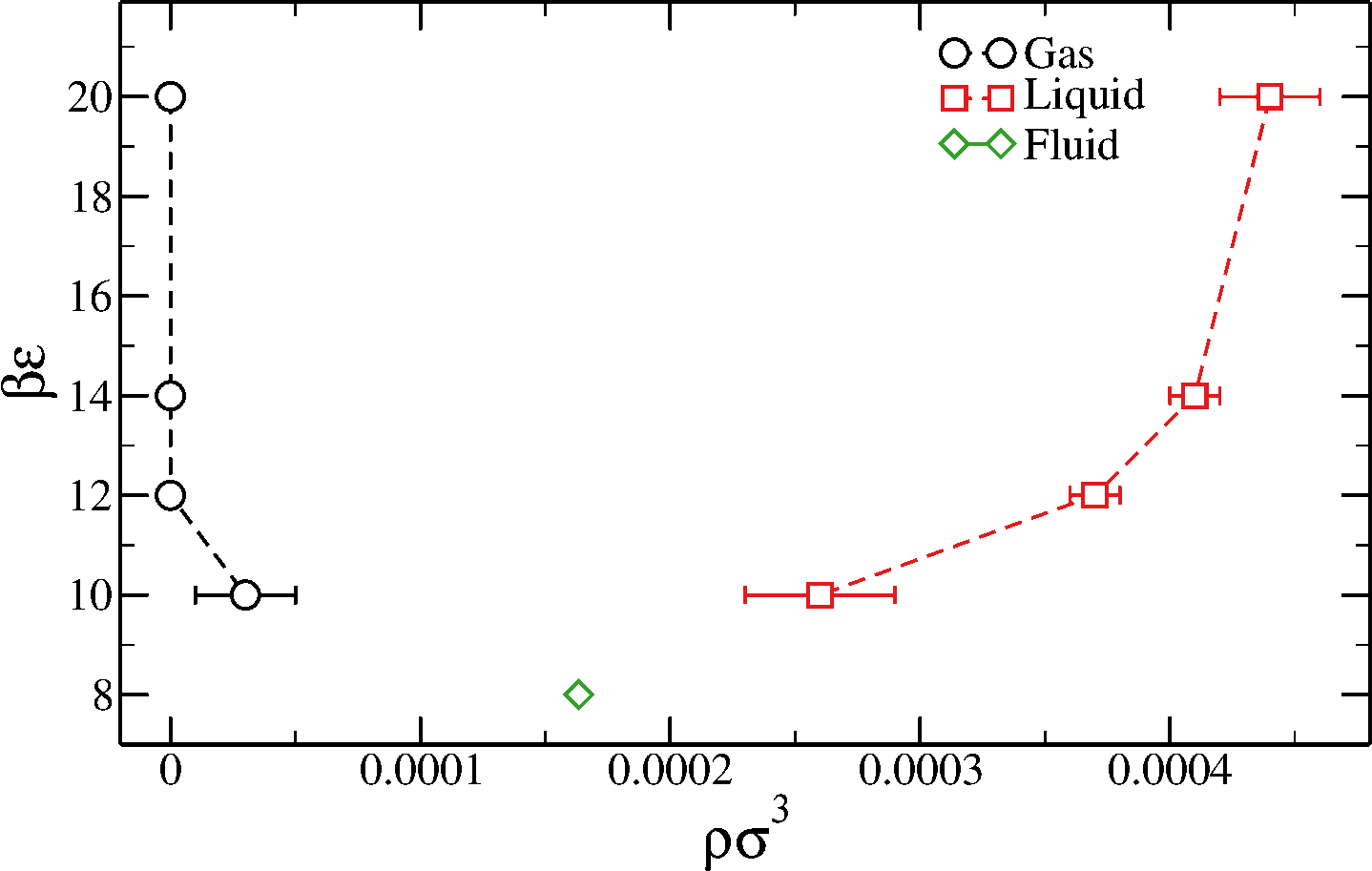
 $T$ is fixed, $\epsilon$ is the depth of the attraction between reactive monomers
$T$ is fixed, $\epsilon$ is the depth of the attraction between reactive monomers
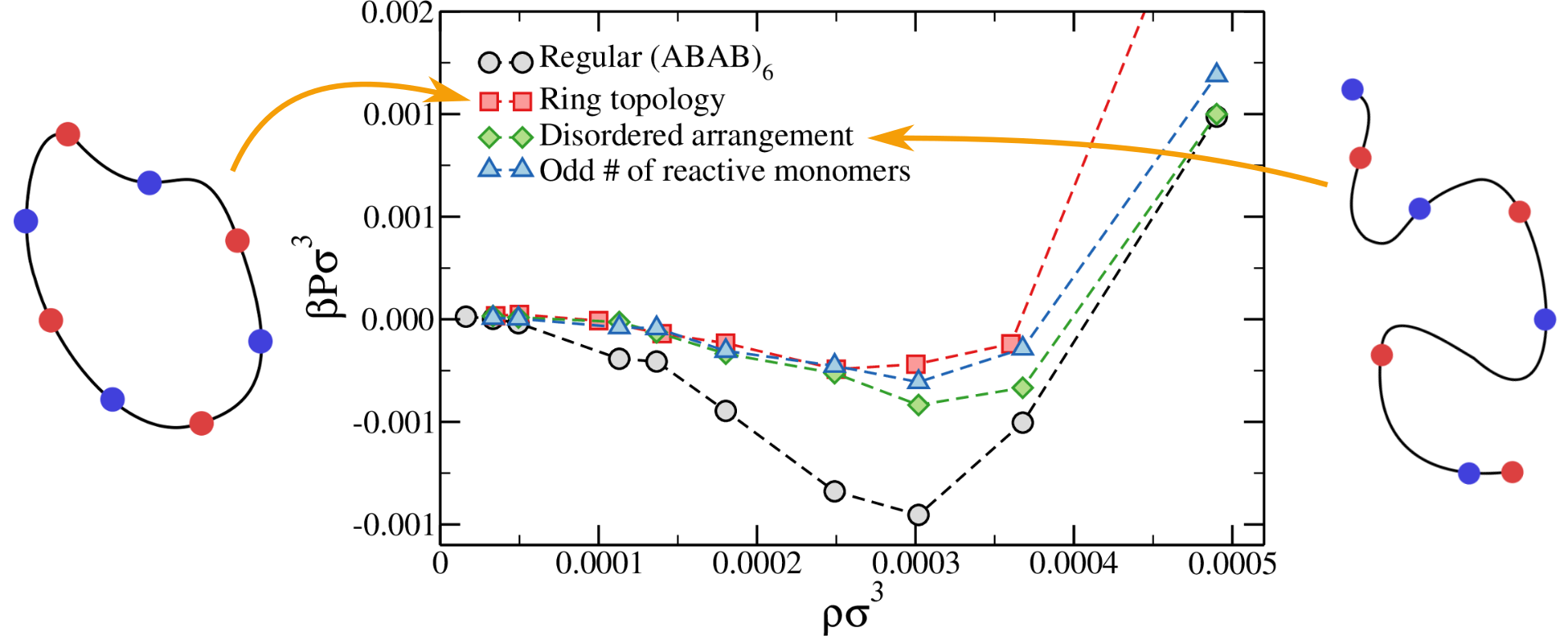
Robustness checks
Phase separation is retained if disordered chains or ring polymers are considered
An interesting biological context where this can be important
Organisms are organised hierarchically

| 1 m | $\approx 10\, \mu$m | $\approx 1\, \mu$m | $\approx 10$ nm |
 |
 |
 |
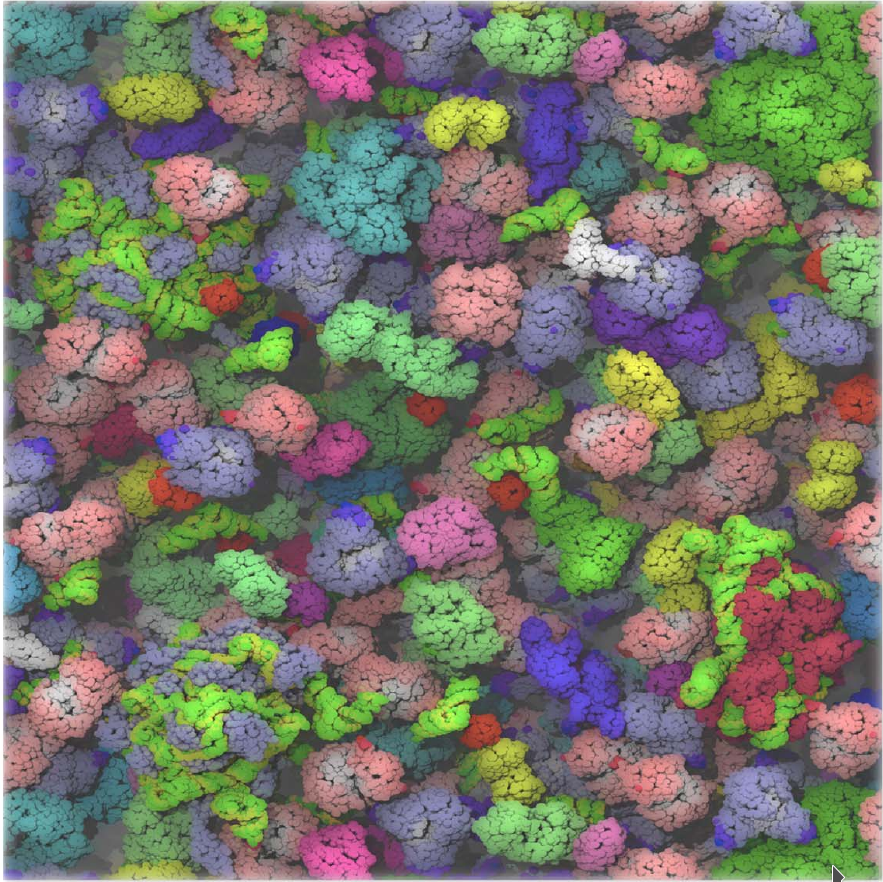 McGuffee and Elcock, PLoS Comput. Biol. 2010 |
Proteins and nucleic acids are polymers!
Organelles
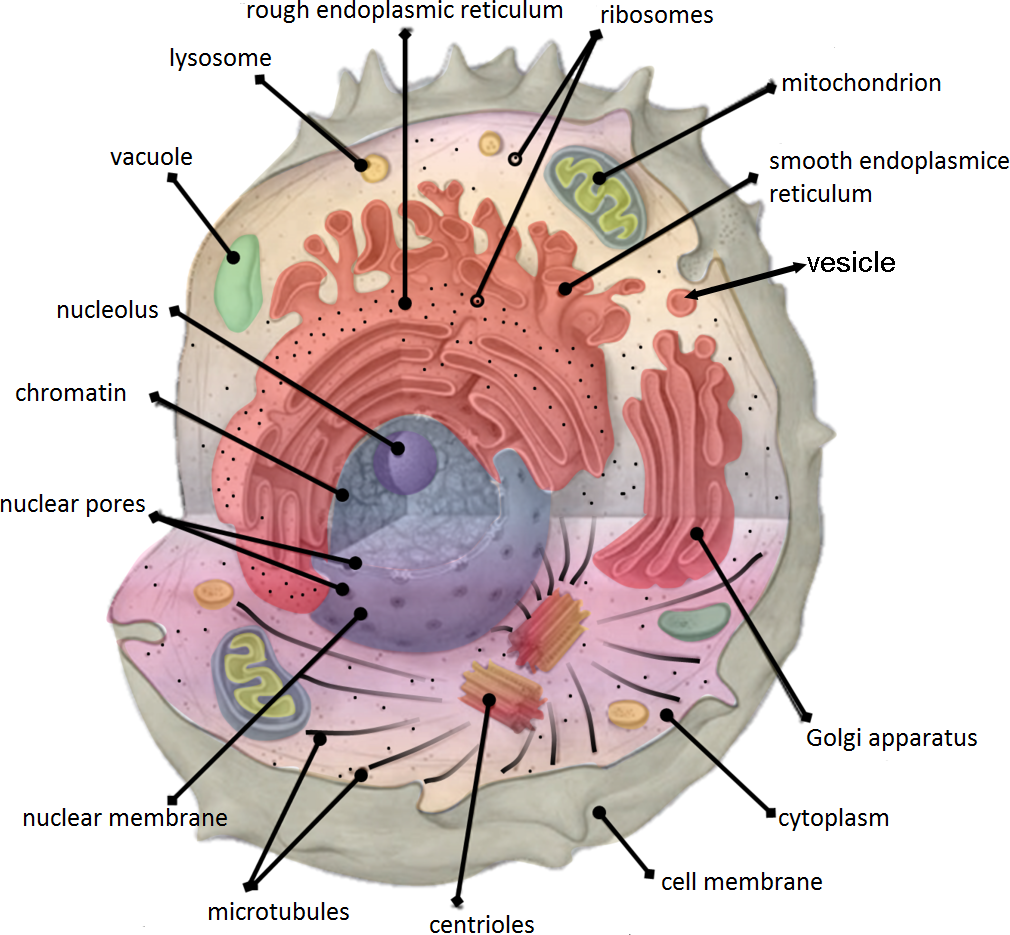
- Perform specialised tasks
- Well-separated from the rest of the cell
- Membrane-bound
- Maintain their "identity" over time
- Communicate through molecular signals
More organelles?

Droplets $\to$ phase separation?
"Liquid-liquid" phase separation (LLPS)
A (gas-like) highly-diluted phase coexist with a (liquid-like) dense phase
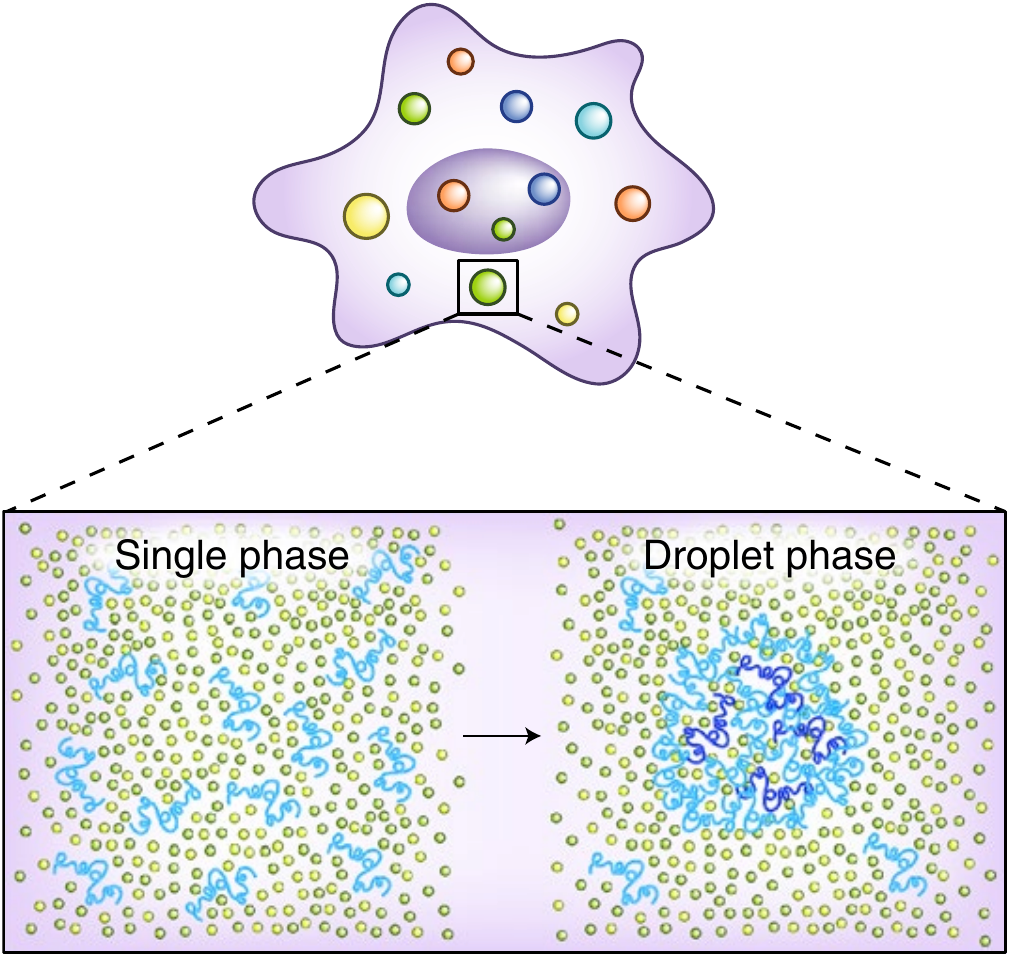 Tang, Nature Methods 2019
Tang, Nature Methods 2019
Membraneless organelles
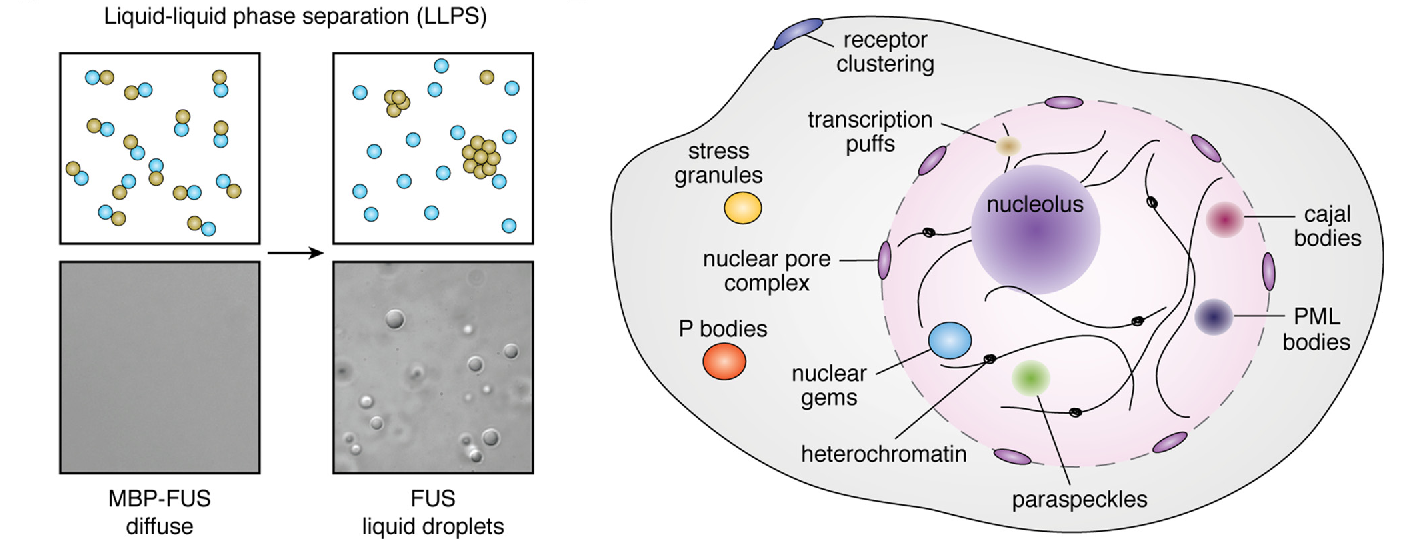 E. Gomes and J. Shorter, J. Mol. Bio. 2018
E. Gomes and J. Shorter, J. Mol. Bio. 2018
- Often called (sometimes improperly) biomolecular condensates
- Liquid-like (they can and do flow, cfr. Brangwynne et al)
- Made of multivalent (intrinsically-disordered) proteins and/or RNA
- Act as reservoirs of biomolecules or as microreactors
- The mechanisms behind their formation are linked to the pathogenesis of several diseases (e.g. Alzheimer's, ALS)
On-the-fly compartmentalisation
- Phase separation relies on a subtle interplay between entropy and enthalpy
- Slightly changing some conditions can suppress/enhance phase separation
- Condensates can quickly adapt to environmental changes!
A visual example
- We take a multi-component mixture $\to$ we need to set many different interactions (red-blue, red-green, green-blue, etc.)
- For this particular choice the resulting system is homogeneous
- We change a single interaction $\to$ phase separation
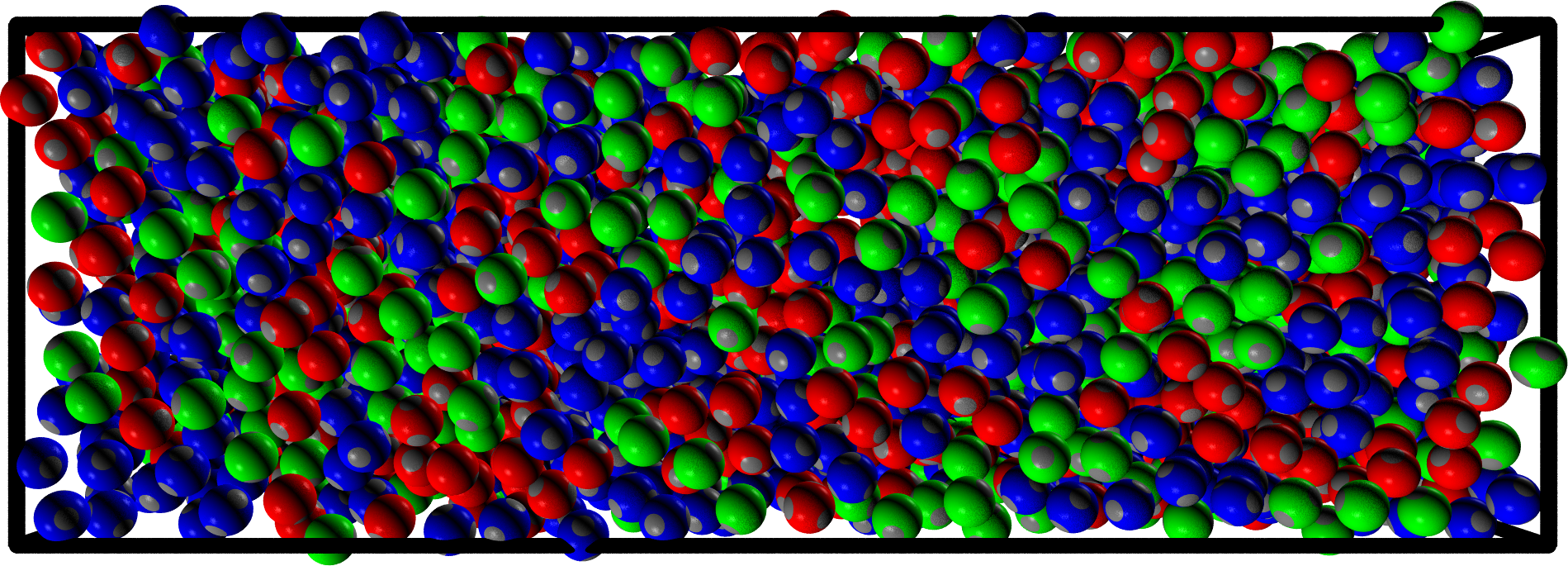
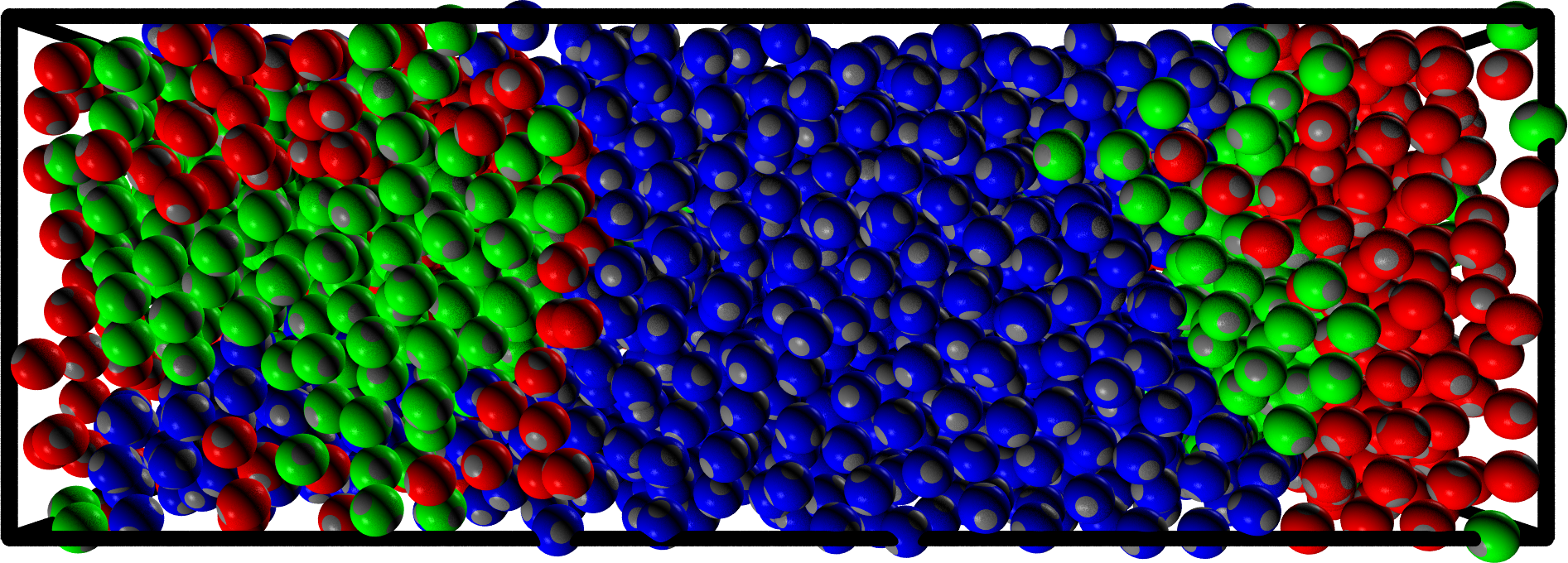
What are these things made of?
- There seems to be many different membraneless organelles in cells
- Composition and behaviour vary greatly from type to type
- Made of proteins interacting through intrinsically disordered regions or lock-and-key mechanisms, and/or RNA
- The main structure is called scaffold and is (usually) formed by phase-separating biomacromolecules
- Other macromolecules interacting with it are called clients
Some evidence?
- The scaffold parts are often (bio)polymers that can bind to themselves or to others
- There is also flexibility, torsional stiffness, different interaction strengths, thermal energy, ...
- Simulations on condensate-forming RNA suggests that "our" entropy plays a role
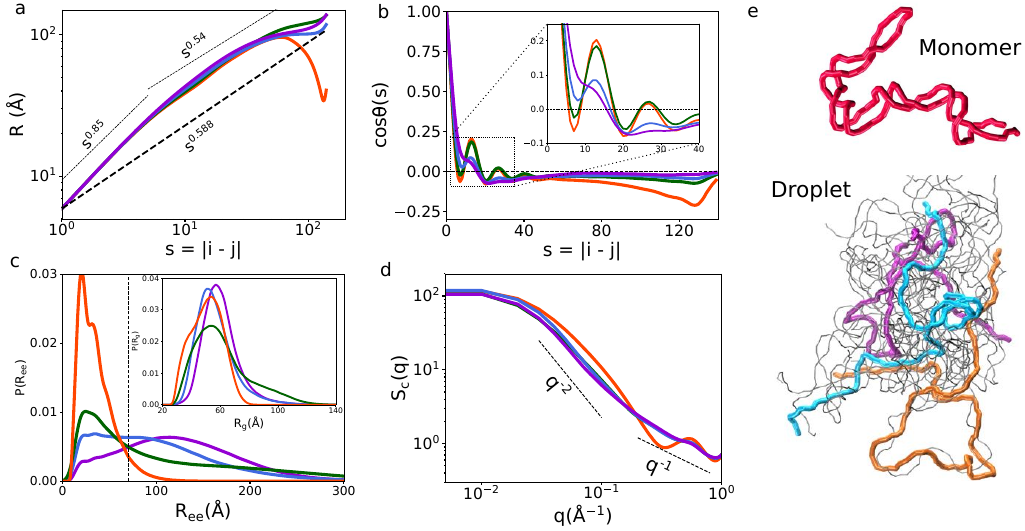 H. T. Nguyen et al, Nat. Chem. 2022
H. T. Nguyen et al, Nat. Chem. 2022
Conclusions & perspectives
- We find a way to controllably tune the behaviour of associative polymers
- The resulting phase behaviour is fully determined by entropy
- The effect may be in play in biological contexts
- Future work: experimental realisation (with DNA?) and dynamics
Thank you!
Questions?
Reference: L. Rovigatti and F. Sciortino, Phys. Rev. Lett. (2022)
Model & methods - details
- Implicit-solvent, coarse-grained description: polymer connectivity (Kremer-Grest) + attraction between the reactive monomers
- Molecular dynamics simulations to compute two-body (effective interactions) and many-body (e.g. pressure) quantities
- A three-body potential to enforce a single-bond-per-site constraint and enable a bond-swapping mechanism to speed-up the dynamics
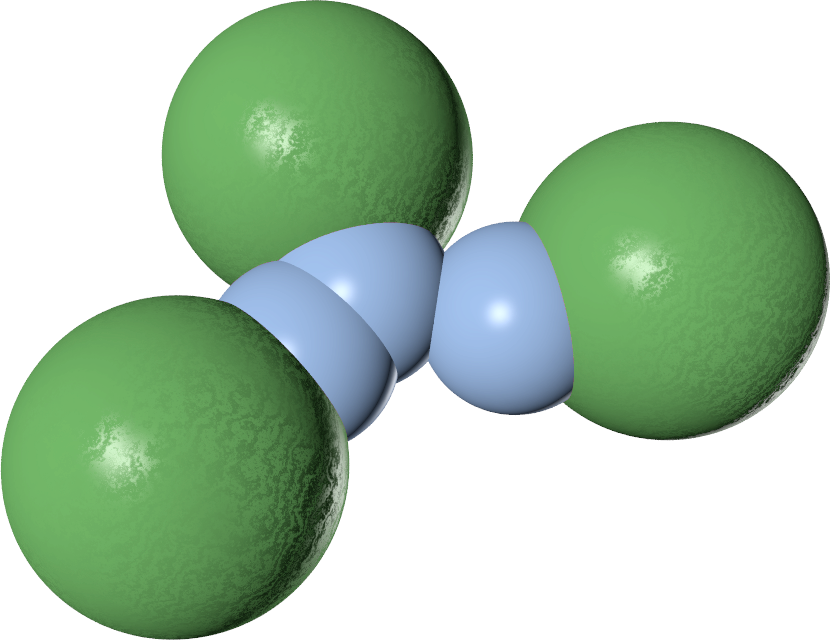
The three-body potential
| Configuration | 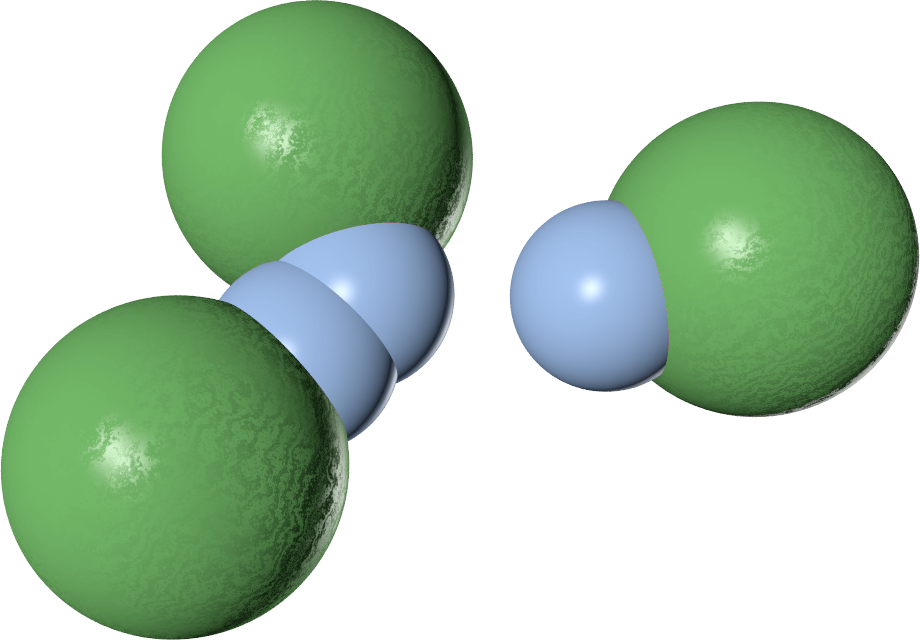 |
 |
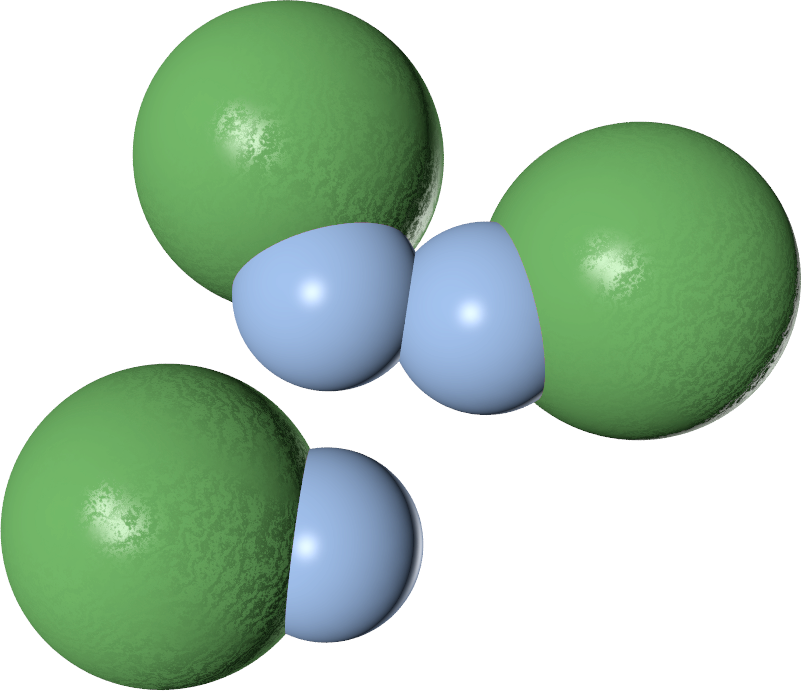 |
| V2 | $-\epsilon$ | $-2\epsilon$ | $-\epsilon$ |
| V3 | $0$ | $\lambda\epsilon$ | $0$ |
| V2 + V3 | $-\epsilon$ | $-2 \epsilon + \lambda\epsilon$ | $\epsilon$ |
The value of $\lambda$ controls the behaviour
- $\lambda \geq 1$ $\to$ single-bond-per-patch
- $\lambda = 1$ $\to$ free swapping!
Weak dependence of the # of bonds and $\beta V$ on the spacing
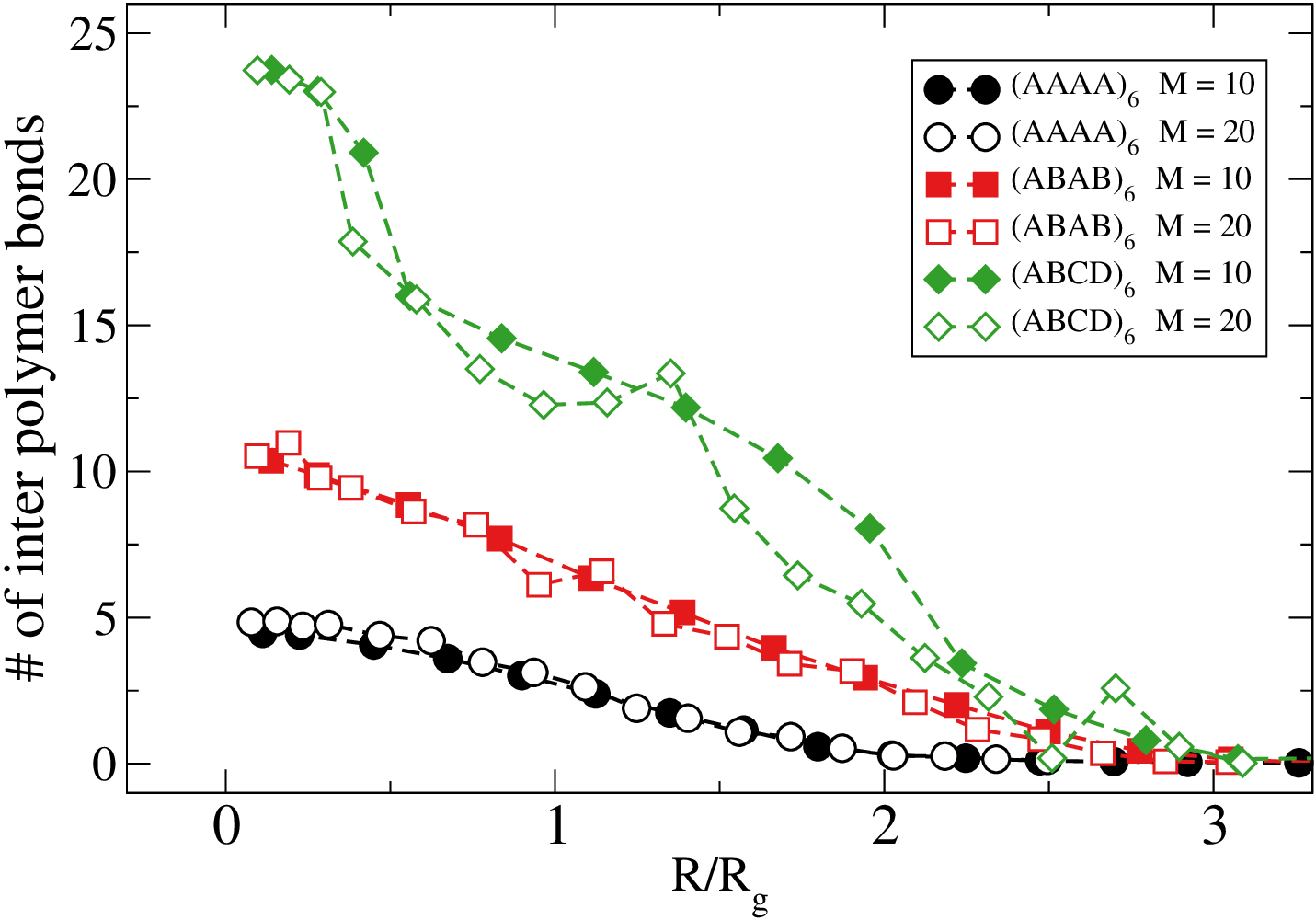
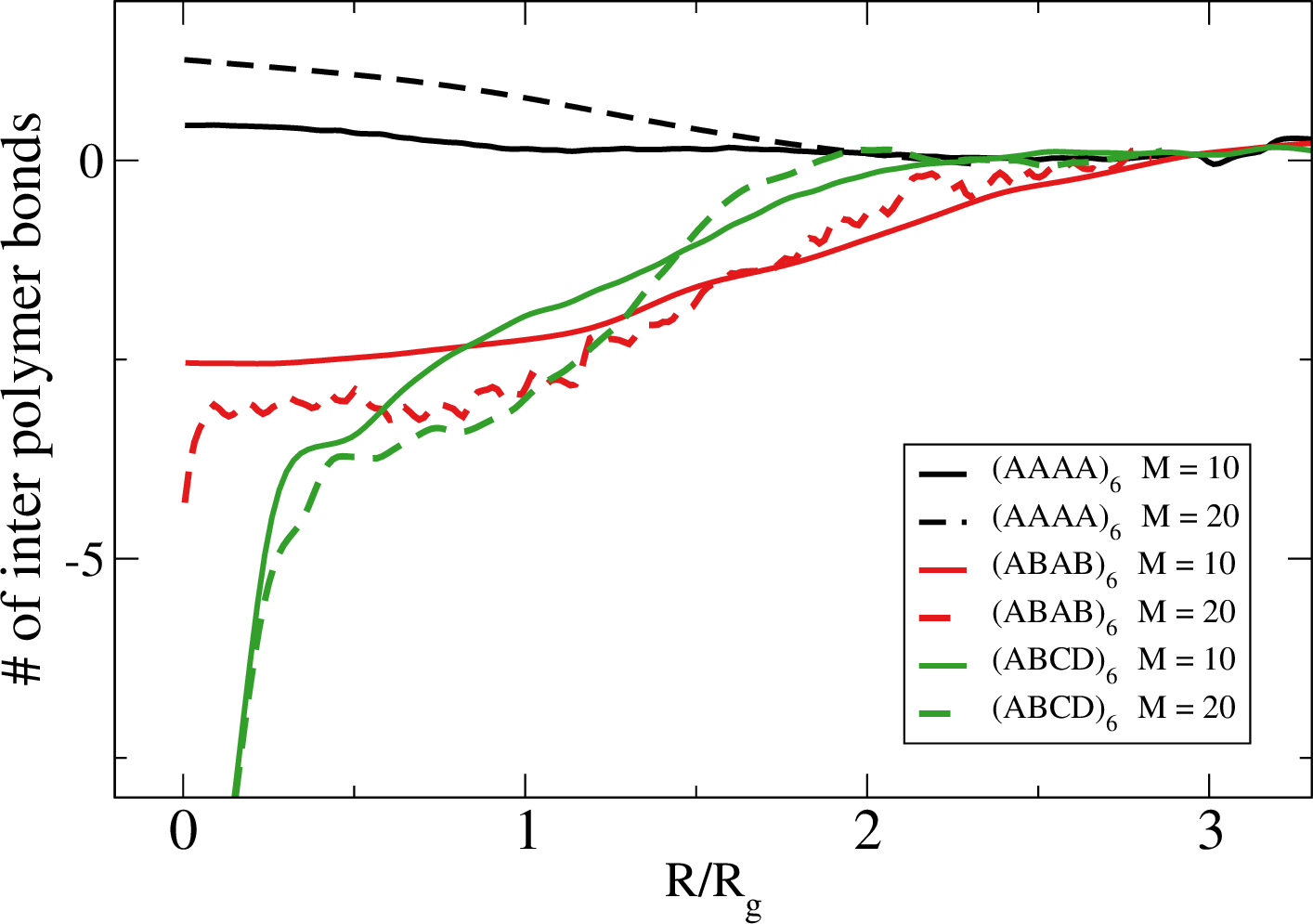 Here $M$ is the number of inert monomer separating two nearby attractive monomers
Here $M$ is the number of inert monomer separating two nearby attractive monomers
The bond-bond autocorrelation function decays to $\approx 0$
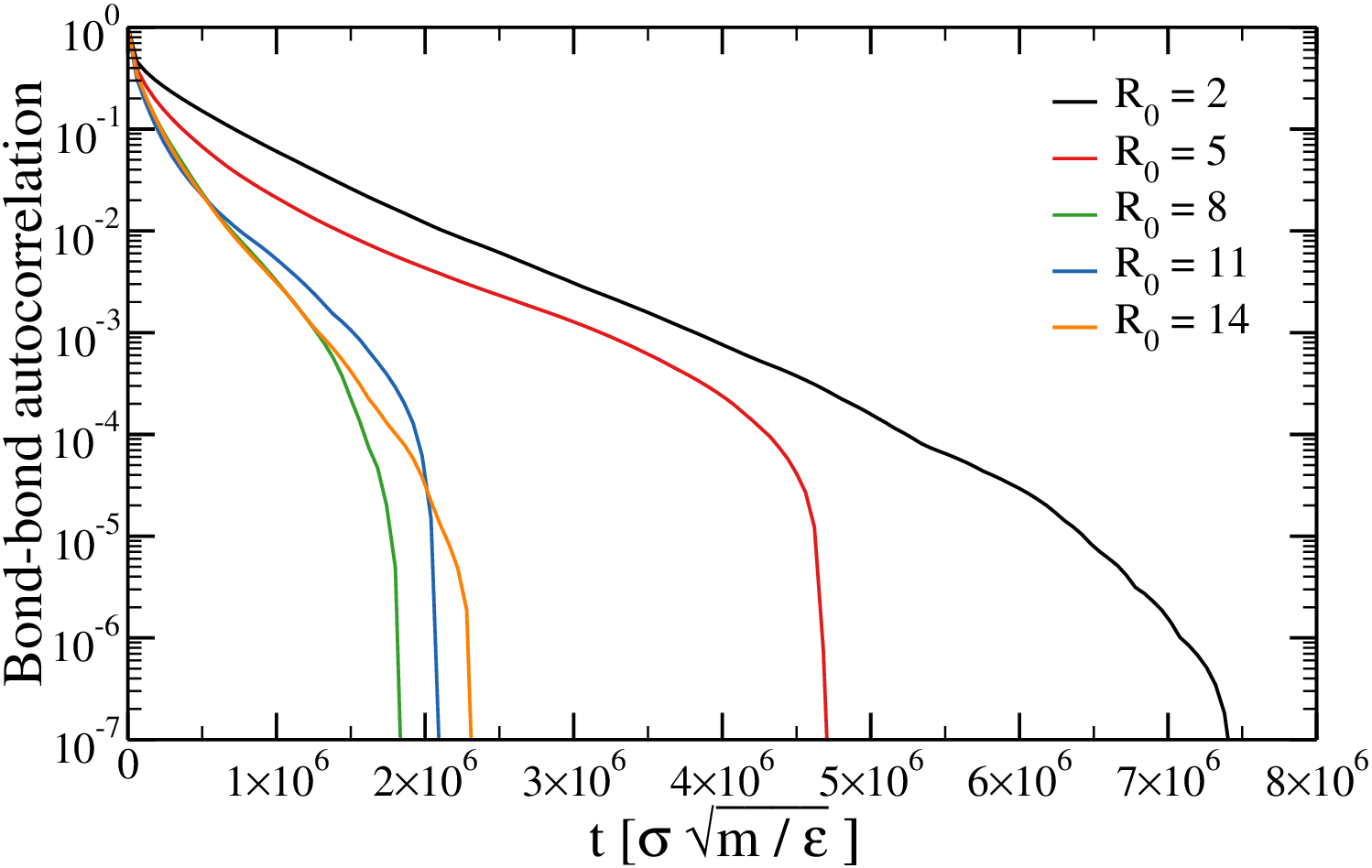
Probability that a bond existing at $t = 0$ is still there at $t = 0$ (two-chain simulations)